The Novatek Experts Blog is our platform to communicate with YOU. Stay up to date with how Novatek actively supports global industry events, contributes to industry journals and publications and collaborates with other leading industry, government and academic professionals. Our goal is to always keep our community informed on the latest Regulatory and Quality Control information around the world and how Novatek actively stays involved in our industry!
All contributions to the Novatek Experts Blog are submitted by leading industry, government, and academic professionals for information and learning purposes. The views expressed by the contributors of Novatek Experts Blog is solely that of the authors.
Doing Digital or Being Digital? How vertical business applications are providing enormous advantages in pharmaceutical manufacturing & quality operations. At Novatek we are committed to excellence in both software and…
DetailsWe are looking forward to seeing our clients at our next User Group Meeting: Closing the Gap: A Holistic Contamination Control Strategy for Annex 1 Hosted by Novatek and Boehringer…
DetailsOn Saturday November 5th Novatek was honored to partner with Hope for Dementia and bring back after 2 years our Annual Charity Gala! The evening was glittering with familiar faces of colleagues,…
DetailsWe are partnering with MilliporeSigma to support the pharmaceutical industry in transferring their microbiological workflows into computerized systems to improve data integrity. Aligning our compliant software solutions with MilliporeSigma’s reliable…
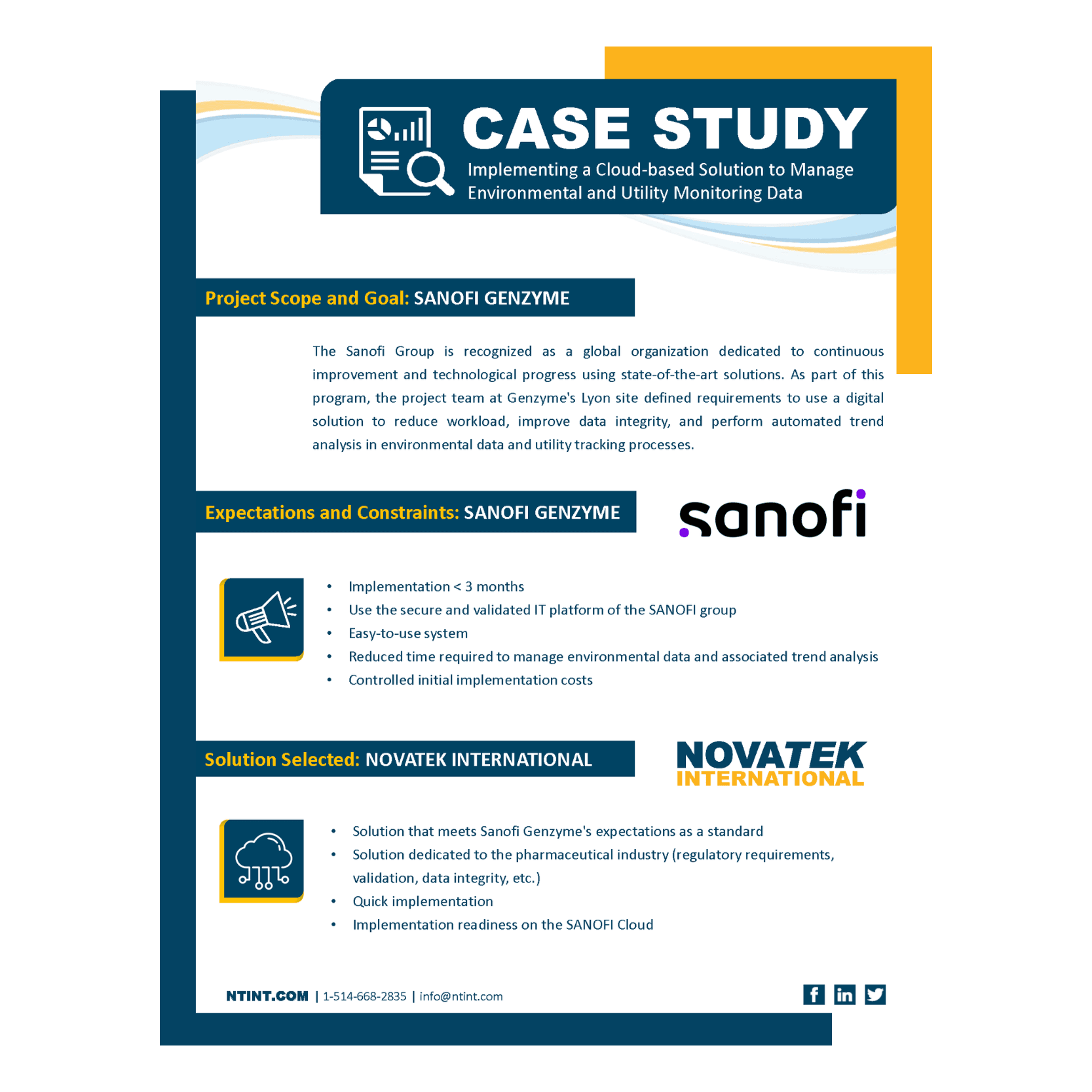
DetailsNovatek International provided Sanofi Genzyme’s Lyon, France pharmaceutical manufacturing site an industry-focused digital solution to improve their contamination control in data integrity, automating trend analysis, and reduced workloads. After a…
DetailsThere are many books that cover Good Manufacturing Practices, however this latest publication on Analytical Testing for the Pharmaceutical GMP Laboratory by WILEY Publishers is by far one of the most concise…
DetailsLeading pharmaceutical company Remedica chooses Nova-LIMS for use at their multiple large scale manufacturing facilities in Cyprus. Novatek is proud to be part of Remedica’s Quality initiatives which are based…
DetailsHow multifaceted, risk-based solutions maximize efficiency, speed, and quality Author: Susan Cleary, Director of Product Development The consequences of contamination in pharmaceutical manufacturing go far beyond compromising patient and consumer…
DetailsNovatek is proud to onboard BioGeneric Pharma as a full quality software portfolio client. BioGeneric Pharma holds high standards in adopting as much automation where they can at their 4000…
DetailsFULL PRESS RELEASE HERE: https://www.newswire.ca/news-releases/novatek-amp-hope-for-dementia-awarded-corporate-citizen-trophy-for-social-commitment-in-the-laurentian-region-quebec-800136504.html MONTREAL, Dec. 16, 2021 /CNW/ – On Friday December 3rd, 2021 Novatek International and it’s charity arm, Hope for Dementia was awarded as a community leader within the…
Details